Child Abuse and Neglect, Sexual Abuse
When most people think of Child,Neglect,Sexual,Abuse, what comes to mind is usually basic information that's not particularly interesting or beneficial. But there's a lot more to Child,Neglect,Sexual,Abuse than just the basics.
Once you begin to move beyond basic background information, you begin to realize that there's more to Child,Neglect,Sexual,Abuse than you may have first thought.
Background
Child sexual abuse (CSA) refers to the use of children in sexual activities when, because of their immaturity and developmental level, they cannot understand or give informed consent. A wide range of activities is included in sexual abuse, including contact and noncontact activities. Contact activities included are sexualized kissing, fondling, masturbation, and digital and/or object penetration of the vagina and/or anus, as well as oral-genital, genital-genital, and anal-genital contact. Noncontact activities include exhibitionism, inappropriate observation of child (eg, while the child is dressing, using the toilet, bathing), the production or viewing of pornography, or involvement of children in prostitution.
The sexual activities are imposed on the child and represent an abuse of the caregiver's power over the child.
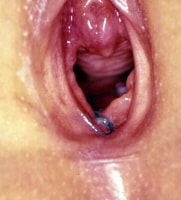
Genital examination of girl in frog-leg supine position after genital trauma. Examination reveals suture in place at 6-o'clock position to stop bleeding from injury. Hymenal edge is irregular and asymmetric. Photo courtesy of Carol D. Berkowitz, MD.
Since the mid 1970s, health care professionals have paid serious attention to sexual abuse of children. Despite the recognition of the clinical importance of sexual abuse of children, some pediatricians may not feel adequately prepared to perform medical evaluations. However, pediatricians are often in trusted relationships with patients and families and are in an ideal position to offer essential support to the child and family. Several paradigms have been proposed to help professionals understand the events that surround the sexual maltreatment of children.
Preconditions for sexual abuse
Motivation of perpetrator: The perpetrator is willing to act on impulses associated with sexual arousal related to children.
Overcoming internal inhibitions: The perpetrator ignores internal barriers against sexually abusing children.
Overcoming external inhibitions: The perpetrator is able to bypass the typical barriers in the caregiving environment that normally serve to impede the sexual misuse of children.
• Overcoming child resistance: The perpetrator is able to manipulate the child to the point of involving the child in the sexual activity.
Longitudinal progression of sexual abuse
Engagement: The perpetrator begins relating to the child during nonsexual activities to gain the child's trust and confidence.
Sexual interaction: The perpetrator introduces sexual activities into the relationship with the child; the perpetrator often begins with noncontact types of activities and, over time, progresses to more invasive forms of contact activities.
Secrecy: The perpetrator attempts to maintain access to the child and to avoid disclosure of the abuse by coercing the child to keep the activities hidden. Coercion to keep the secret can be explicit (eg, threatening the child or the child's family's safety) or it can be implicit (eg, manipulation of the child's trust to create a fear of losing the "friendship" or "attention" should the truth become known to others).
Disclosure: Sexual abuse can become known to others either accidentally, when a symptom from the maltreatment or a third party witnessing the abuse leads to an evaluation, or can be purposeful, as when the child reveals the abuse that is taking place and seeks help.
Suppression: The tumult that occurs after the disclosure prompts the people in the child's caregiving environment to think that they are unable to support the child; thus, these people exert pressure on the child to recant what the child has told in order to go back to the perceived "stable" situation that existed prior to the disclosure.
Sexual abuse typically presents as a pattern of maltreatment that occurs over time.
Traumagenic dynamics model
Traumatic sexualization: The child's sexual feelings and attitudes are shaped in a developmentally inappropriate and interpersonally dysfunctional manner. The child learns that sexual behavior may lead to rewards, attention, or privileges. Traumatic sexualization may also occur when the child's sexual anatomy is given distorted importance and meaning.
The child manifests symptoms of fear, anxiety, and impaired coping.
• Stigmatization: The child's self-image incorporates negative connotations and is associated with words such as bad, awful, shameful, and guilty.
Pathophysiology
The evaluation for suspected sexual abuse may be complicated and is often not straightforward. Frequently, nonspecific behavioral changes are the presenting symptoms prompting an evaluation and leading the health care provider to consider sexual abuse as a possible diagnosis. These nonspecific behaviors are not diagnostic of sexual maltreatment and may be observed in other situations where the child manifests stress as well. Nonspecific behavior changes that warrant consideration of the possibility of sexual abuse may include (1) sexualized behaviors, (2) phobias, (3) sleep disturbances, (4) changes in appetite, (5) change in or poor school performance, (6) regression to an earlier developmental level, (7) running away, (8) truancy, (9) aggressiveness and acting out behaviors, and/or (10) social withdrawal, sadness, or symptoms of depression.
For the purposes of this discussion, the differential diagnosis for each of the following 4 genital findings known to be associated with child sexual abuse is discussed.
Genital bleeding
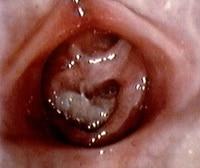
Blood-tinged vaginal or urethral discharge initially may be confused with frank bleeding. Differential diagnoses are as follows:
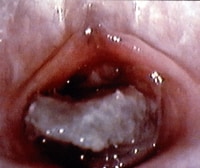
Local factors, such as injury (either accidental or nonaccidental) and/or foreign body irritation (eg, small toy parts, clumped toilet tissue [see the images below]) Prepubertal girl with foul-smelling bloody discharge. The foreign body is lodged in vagina and appears to be toilet tissue that is colonized with bacteria, causing a vulvovaginitis. The foreign body was dislodged with gentle water flushing during examination. Photo courtesy of Carol D. Berkowitz, MD. Genital examination of prepubertal girl with foul-smelling bloody discharge. The foreign body was dislodged with gentle water flushing during examination. Infections, including sexually transmitted diseases (STDs), fungal infections, and/or nonspecific vulvovaginitis
Structural abnormalities, such as vulvar hemangioma
Vaginal discharge
Differential diagnoses are as follows:
Local irritation from abusive sexual contact, foreign body, chemical irritants, and restrictive clothing
Infections, including STDs, fungal infections, nonspecific vulvovaginitis, group A streptococci, Staphylococcus aureus, Haemophilus influenzae, and Mycoplasma species
Physiologic leukorrhea
Anogenital bruising
Differential diagnoses are as follows:
Local injury, including straddle injury, accidental impaling injury, accidental blunt trauma, and abusive injury
Dermatologic conditions, such as mongolian spots, lichen sclerosis, and vascular nevi
Anogenital redness
Local irritation from sexual abuse, poor hygiene, restrictive clothing, and chemicals
Anatomic/structural factors such as perianal fissuring and rectal prolapse
Dermatologic conditions, such as lichen sclerosus, psoriasis, and dermatitis (atopic, contact, seborrhea)
Systemic manifestations of other disorders, such as Crohn disease, Kawasaki syndrome, and Stevens-Johnson syndrome
United States
Prevalence
Professionals conservatively use child sexual abuse prevalence estimates of 20% in women and 5-10% in men. A classic prevalence study of New England male and female college students done by Finkelhor (1984), which used a definition that included both contact and noncontact abuse with older perpetrators and children younger than 17 years, revealed that 19.2% of female students (1 in 5 women) and 9% of male students (1 in 10 men) reported sexual misuse during their childhoods.[4]
Incidence
According to the US Federal Government's official report, Child Maltreatment 2006, approximately 905,000 children were determined to be victims of child abuse; the overall child maltreatment rate was 12.1 cases per 1,000 children.[5] Overall, in 2006, the 905,000 substantiated cases emerged from approximately 3.3 million reports of alleged child abuse and neglect, involving about 6 million children. In addition to the 8.8% of substantiated cases of sexual abuse, an additional 16% were substantiated for physical abuse, and 64.1% were substantiated for child neglect.
In 2009, release of the Fourth National Incidence Study of Child Abuse and Neglect (NIS-4) is expected and eagerly awaited.[6] Prior to the NIS-4's release, older data remain available from the previously congressionally mandated Third National Incidence Study of Child Abuse and Neglect (NIS-3). In 1993, this study reported an estimated sexual abuse incidence rate of 3.2 cases per 1000 children (or a total of 217,000);[7] this represented 29% of the total number of children known to have been abused. NIS-3 used a definition that subsumed a range of behaviors, including intrusion, genital molestation, exposure, inappropriate fondling, and unspecified sexual molestation.
At present, the NIS-3 is the single most comprehensive source of information about the current incidence of child abuse and neglect in the United States and is based on a nationally representative sample. With sexual abuse, the number of undisclosed incidents is believed to be large due to the stigma and criminal behavior involved.
The 1993 NIS-3 incidence figure of 3.2 cases per 1000 children represents a statistically significant (68%) increase from the 1986 Second National Incidence Study of Child Abuse and Neglect (NIS-2) incidence of 1.9 per 1000 children. In part, this difference is due to increased recognition of sexual abuse in the pediatric population. Finkelhor and Jones (2008) at the Crimes Against Children Research Center have been tracking the trends in child maltreatment statistics collected by the Federal Government and have found a national decline in the incidence of both physical and sexual abuse that began in the middle the 1990s and continues through the early 2000s.[8]
However, no decline was found in the rate of child neglect. Specifically, child sexual abuse substantiations have seen a 53% downward trend from the peak annual incidence observed in 1992 (see image below). From 2005-2006, substantiated child sexual abuse cases declined 5%.
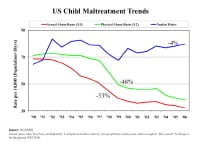
US child maltreatment trends.
Finkelhor and Jones have explored the potential reasons for the decline in child sexual abuse cases and have focused on factors that may be impacting the actual incidence as well as factors that may be influencing the reporting and investigation of reported cases, which may then downstream impact the number of substantiated cases (see image below).
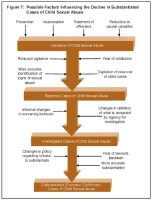
Possible factors influencing the decline in substantiated cases of child sexual abuse.
Optimistically, prevention efforts, incarceration, and treatment of perpetrators (along with other societal factors) may actually be decreasing the number of children who are harmed by sexual abuse. Numerous psychological and medical consequences have been described as associated with sexual abuse. Psychological disorders are reported as having an increased incidence in those who have been abused sexually and include depression, eating disorders, anxiety disorders, substance abuse, somatization, posttraumatic stress disorder (PTSD), dissociative disorders, psychosexual dysfunction in adulthood, and numerous interpersonal problems, including difficulties with issues of control, anger, shame, trust, dependency, and vulnerability.
PTSD and its relationship to sexual abuse have received considerable professional attention. Note that no universal short-term or long-term impact of sexual abuse has been identified, and the presence or absence of various symptoms or conditions does not indicate nor disprove the occurrence of sexual abuse.
Medical sequelae of sexual abuse include numerous medical conditions, including functional GI disorders (eg, irritable bowel syndrome, dyspepsia, chronic abdominal pain), gynecologic disorders (eg, chronic pelvic pain, genital or anal tears), and various forms of somatization involving neurologic conditions and pain syndromes. Additionally, children may contract STDs via sexual abuse, and postpubertal females may become pregnant.
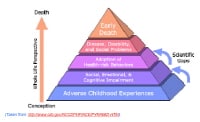
In groundbreaking work, Felitti et al have explored the connection of exposure to childhood abuse and household dysfunction to subsequent health risks and the development of illness in adulthood in a series of studies referred to as the Adverse Childhood Experiences (ACE) studie s.[9] In order to assess exposure to child abuse and neglect, the ACE questionnaire asked about categories of child maltreatment, specifically psychological, physical, and sexual abuse. When asking about sexual abuse, the questionnaire asked the patients if an adult or person at least 5 years older then had ever (1) touched or fondled them in a sexual way; (2) made them touch the adults or older person’s body in a sexual way; (3) attempted oral, anal, or vaginal intercourse with them; or (4) actually had oral, anal, or vaginal intercourse with them. In order to assess exposure to household dysfunction the ACE questionnaire asked questions by category of dysfunction, such as having a household member who had problems with substance abuse (eg, problem drinker, drug user), mental illness (eg, psychiatric problem), or criminal behavior (eg, incarceration) and having a mother who was treated violently.
In addition to the questionnaire information, the standardized medical examination of the adult assess risk factors and actual disease conditions. The risk factors included smoking, severe obesity, physical inactivity, depressed mood, suicide attempts, alcoholism, any drug abuse, a high lifetime number of sexual partners, and a history of STDs. The disease conditions included ischemic heart disease, cancer, stroke, chronic bronchitis, emphysema, diabetes, hepatitis, and skeletal fractures. Once all of the data were collected and analyzed, Felitti et al reported that the most prevalent ACE was substance abuse (25.6%), the least prevalent ACE was criminal behavior (3.4%), and the prevalence of sexual abuse was 22%.
Risk of alcoholism, drug abuse, depression, and suicide attempt increased 4-12 fold
Rates of smoking, poor self-rated health, and high number of sexual partners and STDs increased 2-4 fold
Physical inactivity and severe obesity increased 1.4-1.6 fold
Adverse Childhood Experience (ACE) Pyramid.
Race
This initially may be surprising due to the disproportionate overrepresentation of children of color who are involved with the child welfare system. NIS-3 data were consistent with the 1986 NIS-2 findings, which also failed to demonstrate any evidence of disproportionate victimization in relationship to children's race. Finkelhor has concluded that race, ethnicity, and social class do not appear to be associated with risk of child sexual maltreatment.
Gender differences are noted in the reported incidence of sexual abuse. In the NIS-3, a statistically significant difference was noted, with girls experiencing sexual abuse at more than 3 times the rate of boys (4.9 per 1000 girls compared with 1.6 per 1000 boys). Child Maltreatment 2006 did not separately report the number of sexual abuse cases by gender.[5]
Age
Age differences are observed in the reported incidence rates of sexual abuse for children aged 0-2 years (incidence is 1 per 1000) compared with children aged 12-14 years (incidence is 2.6 per 1000) and children aged 15-17 years (incidence is 2.7 per 1000). Incidence rates of sexual abuse in children aged 3-11 years widely varied and made the statistical comparisons unreliable. Of the approximately 78,000 children for whom age data are reported in Child Maltreatment 2006, the age breakdown shows that 6% of children who were sexually abused were younger than 4 years, 22% were aged 4-7 years, 23% were aged 8-11 years, and 47% were aged 12 years or older
So now you know a little bit about Child,Neglect,Sexual,Abuse. Even if you don't know everything, you've done something worthwhile: you've expanded your knowledge.
 Atrial Flutter in Children
Atrial Flutter in Children  Electrocardiogram Interpretation in Children
Electrocardiogram Interpretation in Children 
 Subscribe to email feed
Subscribe to email feed



